Pivot Point International, Inc. SFE Textbook changes Page 19
c h e m i s t r y
223
7
Molecules
When unstable atoms combine chemically by
sharing electrons, a molecule is formed. A
molecule is the smallest particle of a compound
that has all the chemical properties of that
compound. Molecules are two or more of the
same atoms joined together by one or more
chemical bonds.
Atoms in a molecule are described using the
chemical symbols for each element (such as C
or O), sometimes accompanied by a subscripted
number indicating the number of atoms of
each element. For example, the symbol for
oxygen is O2. This means the molecule has
two oxygen atoms. Another example is ozone,
with the symbol O3, that consists of three
oxygen atoms. These are examples of fairly
simple molecules.
Compounds
When the atoms that combine are different (for
example, an atom of hydrogen and an atom of
oxygen) the resulting molecule is a compound.
Compounds are created by chemically uniting
two different elements. Compounds formed by
the union of individual elements have their own
unique chemical and physical characteristics.
Many products
estheticians use are the
result of the chemical
uniting of two different
elements or the making
of compounds. The
reactions that occur in
the outer shell of atoms
eventually leads to the
composition of the
appropriate ingredients
for clients' specific
skin care needs.
Salon Fundamentals
TM
Esthetics
+
=
Did you know that CO2 is found in soft
drinks? Carbon dioxide gives soda
its carbonation.
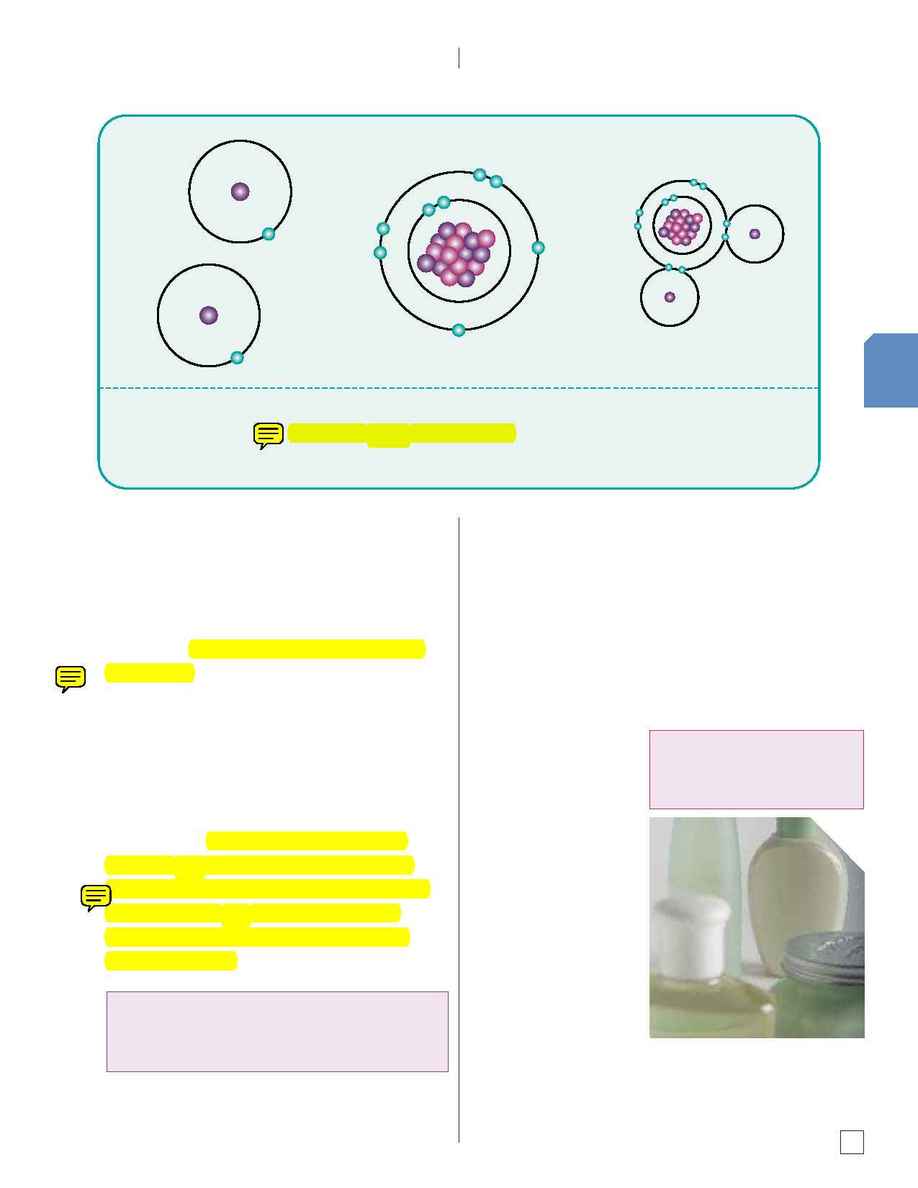
When two hydrogen atoms, each with one electron, combine with one oxygen atom and its eight
electrons, the result is the compound H2O, which is water. This diagram shows two gases--hydrogen
and oxygen--uniting and becoming a liquid.
Scientists have identified
more than four million
chemical compounds.
Hydrogen
Oxygen
H2O
