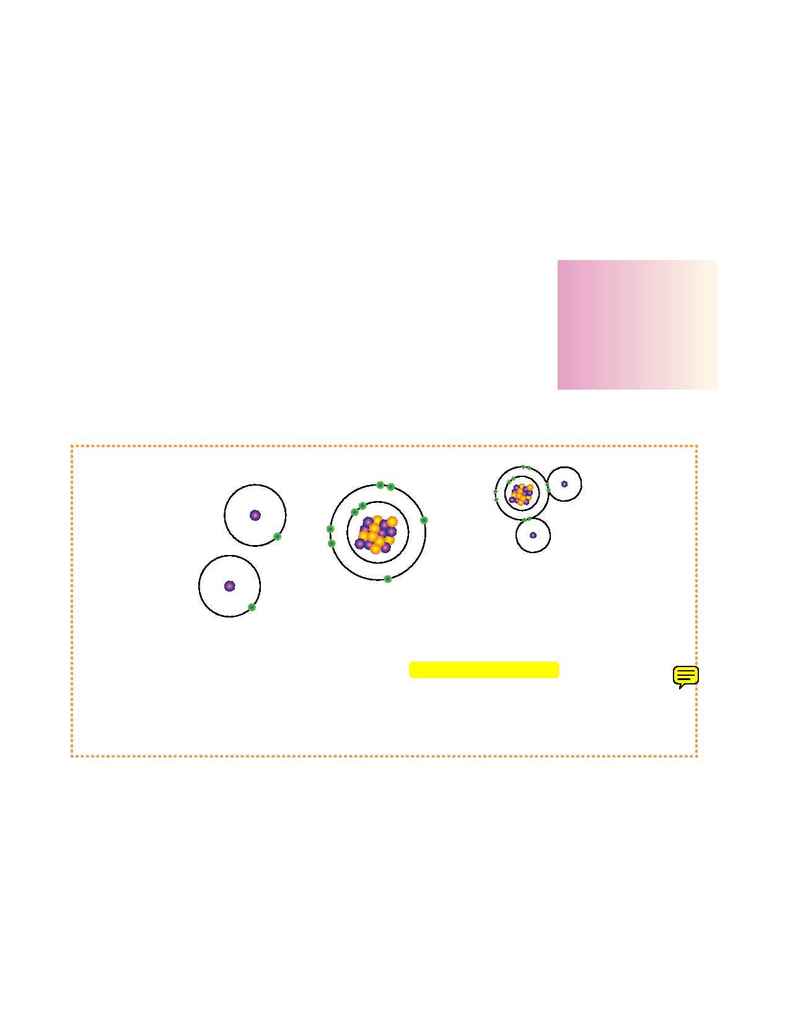
The chemical behavior of an atom depends mostly on the number of electrons in its outermost orbit-
ing path or shell. Some atoms by their very structure are not missing any electrons in their outer
shell. These atoms are considered stable and are electrically neutral.
If the outer shell of the atom is missing electrons, however, the atom is considered unstable or reac-
tive. Unstable atoms seek other atoms with which they can share electrons to complete their outer
shell. When they combine, they make more complex units, known as molecules.
Molecules
When unstable atoms combine chemically by sharing electrons, they form
molecules.
A molecule is two or more atoms joined together by a
chemical bond.
If the atoms that combine are different, for example, an
atom of hydrogen and an atom of oxygen, the resulting molecule is a
compound. Different atoms joined together as molecules become the
smallest parts of a compound.
Chemical Bonds
You are now somewhat familiar with the diagrams of the atoms of the five elements of hair carbon,
oxygen, hydrogen, nitrogen and sulfur. These atoms combine chemically to create compounds that
eventually create the protein of hair. To see how they join to form hair, you'll need to learn a little
about chemical bonds, starting with amino acids.
114
SALON FUNDAMENTALS
+
=
When two hydrogen atoms, each with one electron, combine with one oxygen
atom and its eight electrons, the result is a water molecule of the compound
H
2
O. This is an example of two gasses hydrogen and oxygen uniting and
becoming a liquid.
With an element,
the atoms are
the same. With
a compound,
the atoms are
different.
