2
The boiling points of the C
8
aromatic isomers are very close. However, orthoxylene can be
separated from the feed mixture by intense distillation. Ethylbenzene can only be removed
by expensive superfractionation. Although there is a good demand for EB to produce
styrene, most of the reformate-based EB is converted into benzene or xylenes in the
isomerization unit. The para- and meta- isomers cannot economically be separated by
distillation.
Conversely, the wider range of freezing points led early scientists to crystallization as the
method of choice for PX recovery. A large segment of the industry profitably uses
crystallization as the primary method for paraxylene production.
Technology Developments
Perhaps the most noteworthy development in paraxylene technology during the 1970's and
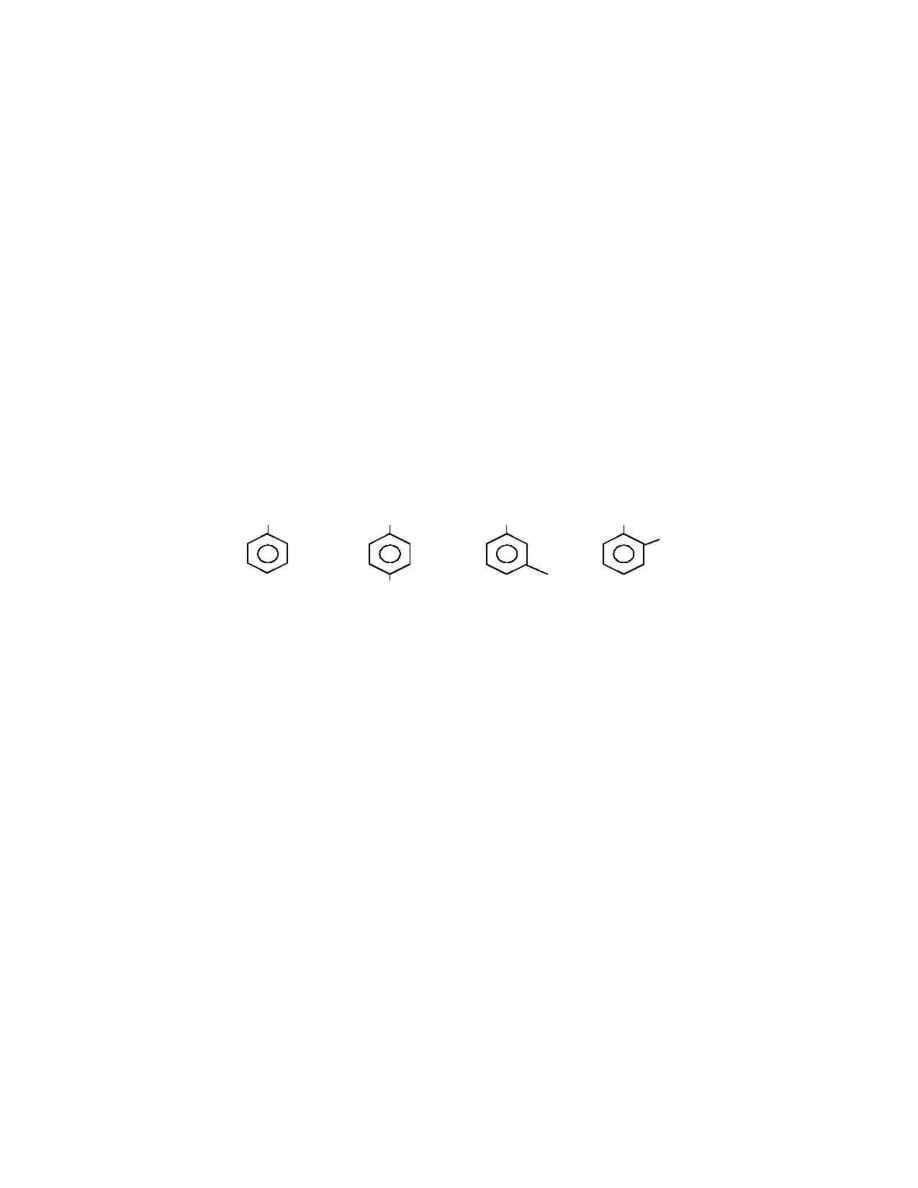
1980's has exploited another characteristic of the xylenes, which is the molecular size. As
you can see below, the C
8
aromatic isomers have the alkyl groups at different positions,
giving them different molecular diameters.
The molecules can be separated by selective adsorption onto a molecular sieve. Such
processes are well known in the industry.
1
Another interesting innovation has been the selective disproportionation of toluene (which
has a molecular diameter similar to paraxylene) into benzene plus xylenes. This method uses
a shape-selective zeolite catalyst, which permits the para-xylene isomer to be preferentially
produced. Because the xylenes are enriched in paraxylene, it is easier and less expensive to
purify the product.
Assessment of Current Processes
The industry has been enamored of the adsorption process for paraxylene recovery, because it
provided a real improvement over the early generation crystallization processes. There are
some aspects of the selective adsorption process, which are advantageous over crystallization
methods. However, when compared at the same unit capacity and current state of the art, the
overall production costs of paraxylene using adsorption processes are higher than using
modern crystallization.
Crystallization has been often misunderstood or mischaracterized as an outdated technology
for paraxylene recovery. Early versions of this technology relied on small-scale, low-
reliability equipment, which was arranged in multiple processing trains. Consequently
crystallization processes were deemed to be maintenance intensive. This is no longer true.
M o l e c u l a r S t r u c t u r e o f C
8
A r o m a t i c s
C
2
H
5
C H
3
C H
3
C H
3
C H
3
C H
3
C H
3
E B
P X
M X
O X
