Kolmetz.com Innovations in Paraxylene Technology Page 2
1
Table 2. Physical properties of the C
8
aromatic isomers
Normal
Boiling
Point, ░C
Freezing
Point, ░C
Ethylbenzene
136.0
-95.0
Paraxylene
138.3
+13.2
Metaxylene
139.1
-47.9
Orthoxylene
144.3
-25.2
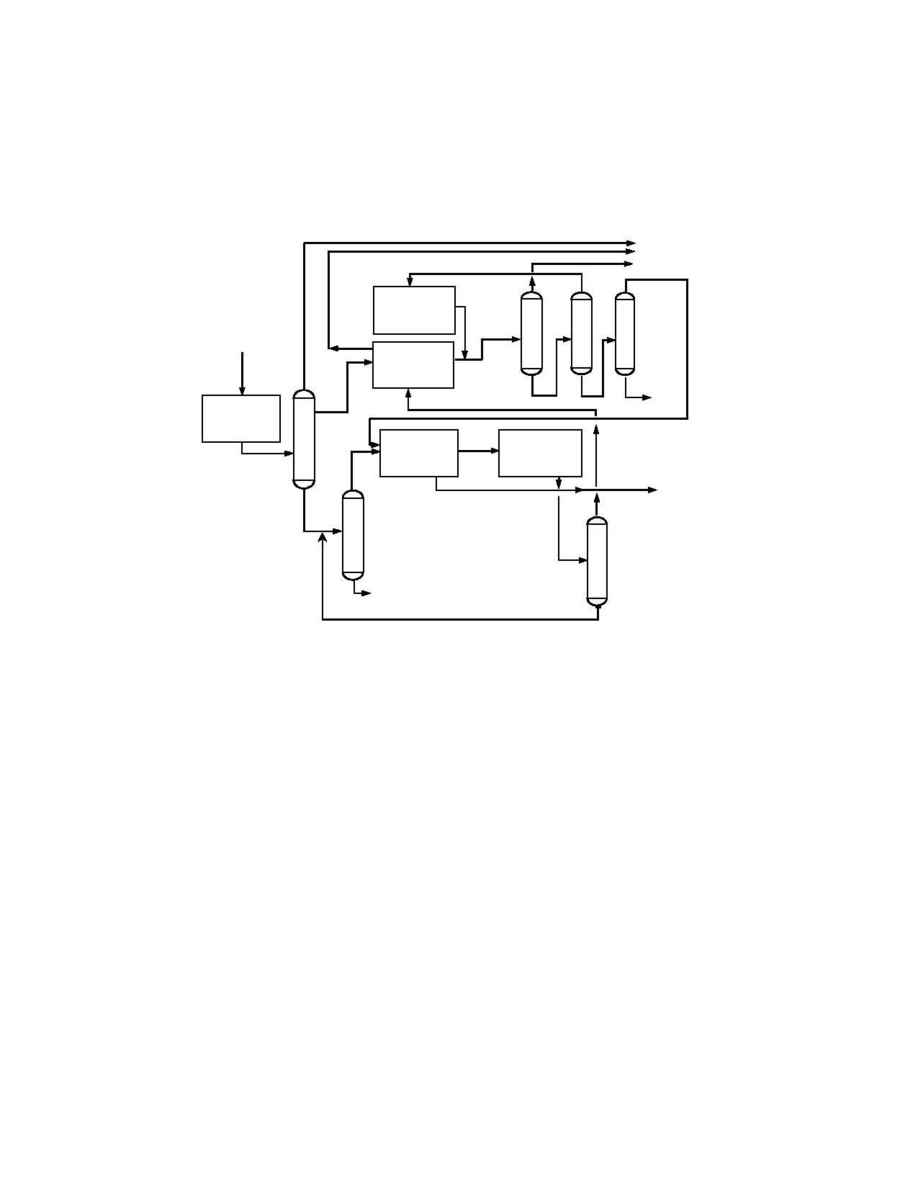
Figure 1 below shows a typical naphtha-based aromatics complex producing paraxylene and
benzene, illustrating the arrangement of the paraxylene recovery and isomerization units.
Figure 1. Typical aromatics complex
The reformed naphtha is first separated into a light and heavy fraction. The light fraction
contains the benzene and toluene fractions, which are usually purified by solvent extraction.
If the reformer severity is high enough, the C
8
cut from the heavy fraction can be made by
heart cut distillation. The C
8
's go to the paraxylene recovery section, and the remaining
isomers are routed to the isomerization section. All remaining streams are recycled to
extinction or routed to gasoline blending.
Table 2 below shows the primary physical properties of the xylenes, which affect the
processing and separation methods used in the complex.
Paraxylene
Recovery
Aromatics
Extraction
Toluene
Conversion
Reformate Splitter
Benzene Column
Toluene Column
Xylene Column
Xylene Column
Deheptanizer
Benzene
C
6
-C
7
raffinate
C
9
+
Paraxylene
Hydrotreated
Naphtha
C
9
+
Xylene
Isomerization
Catalytic
Reformer
C
5
