5
Ligands
The enantiomeric excess (ee) for different rhodium-phosphine ligands has been compared for the
asymmetric catalysis in the equation:
RCH+C(NHCOCH
3
)COOH RCH
2
C*H(NHCOCH
3
)COOH RCH
2
C*H(NH
2
)COOH
(1)
It is shown that DIPAMP (Di-o-methoxyphenyl) has highest ee. among the other ligands.
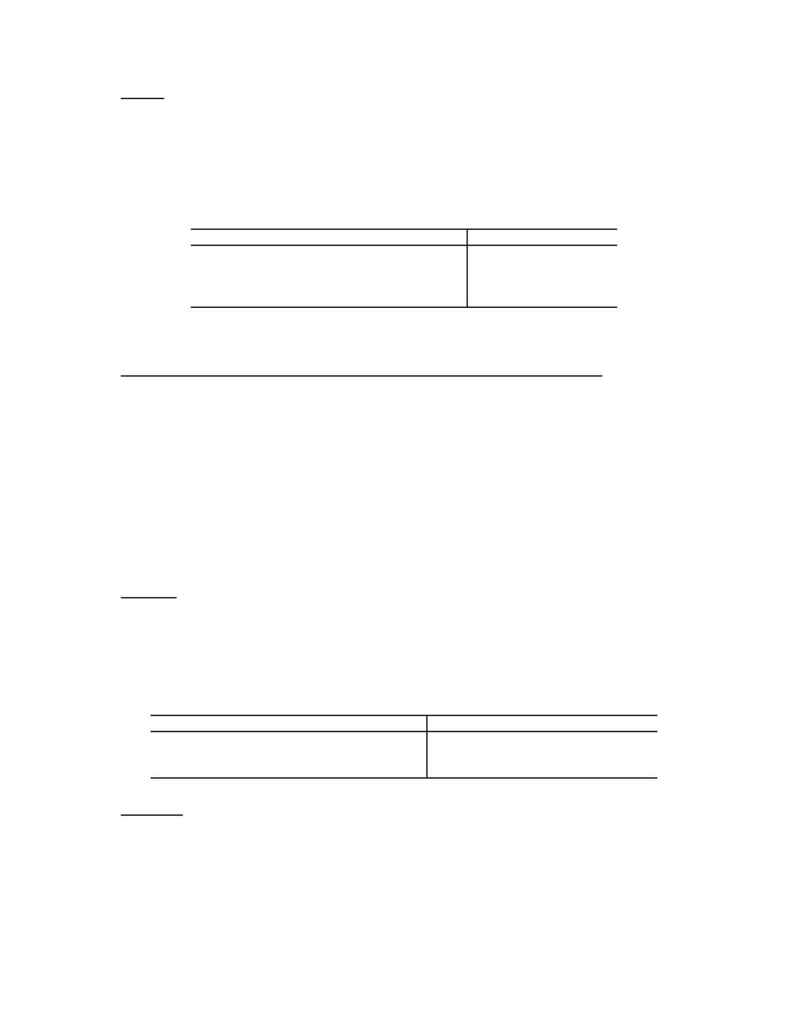
Table 1. Genesis of Phosphines
Ligands Enantiomeric
excess
Methylpropylphenylphosphine 28%
o-methoxyphenyl (PAMP)
50-60%
Cyclohexyl-o-anisylmethylphosphine (CAMP)
80-88%
Di- o-methoxyphenyl (DIPAMP)
95%
Some other phosphine with relatively high %ee (more than 80%ee) on (Z)-
-acetamidocinnamic are R-
CAMP, R,R-DIOP, R,R-DIPAMP, Chiraphos, BPPFA, BPPM, Rhone-Poulenc and PNNP.
Reaction Conditions: Pressure, Temperature, catalyst poison, safety, chiral multiplication
Usual conditions of reaction are about 3 atm pressure and 50
o
C with about 1000/1 mol ratio of substrate
to catalyst in aqueous ethanol or 2-propanol. Generally, higher alcohols give marginally better efficiency
than methanol. In all cases the efficiency drops with pressure. This problem can be resolved using
triethylamine or other base to generate anion, and the slower hydrogenation rate due to the base can be
offset by running at 25-50 atm. In some instances with the anion it is only possible to get high ee's at 0
o
C
where reaction rates are impractically slow. The hydrogenation is poisoned by oxygen or peroxides.
These impurities must be removed for efficient catalyst usage. One advantage of the homogeneous
asymmetric hydrogenation compared to heterogeneous is it does not catalyze the reaction of hydrogen or
solvent vapors with oxygen thus not pyrophoric. This property is important in operational safety. This
catalyst in the absence of oxygen or peroxide are very active, it is easily possible to make thousands of
moles of product per mole of chiral agent. This enormous multiplier effect easily offset the high cost of
the catalyst.
Substrates
The behavior of above mentioned ligands on (Z)-
-acetamidocinnamic will change once varies the
substituenst on the olefin. Whenever an olefin substrate has the capability of forming chelate with a metal,
then DIPAMP is the most generally applicable ligand. If considering simple olefins such as
-
phenylacrylic acid or substituted prochiral styrenes, then DIOP is the best choice. The changes and the
effect is show in Table 2.
Table 2. Ligands behave to different substrates
Changes Effects
Carbonyl group change to ester, amide, or nitrile DIOP inefficient
Aromatics substituent change to aliphatic
Only DIPAMP is efficient
Carbonyl group change to trifluoromethyl group Only DIPAMP is efficient
Mechanism
The chiral phosphine and the hydrogen must all be on the metal at the same time. The catalyst must show
a considerable preference for adding hydrogen to the re face of the olefin. Figure 2 summarized the
mechanism of hydrogenation. It is observed that the stereochemical control occurs at either the hydrogen
addition (XXI) or the rhodium alkyl hydride (XXII) stage. The greater ease of forming XXII with its re
face on the metal rather than the si face provides a reasonable explanation of the steric control.
H
2
cat
