2
MCA is the desired product, DCA is the undesired by-product and the intermediate product acetyl
chloride is the catalyst. In order to maximize the selectivity of MCA, the concentration of acetyl chloride
and MCA in the reactor and the still need to be closely monitored. Table 1 is the concentrations range for
each components.
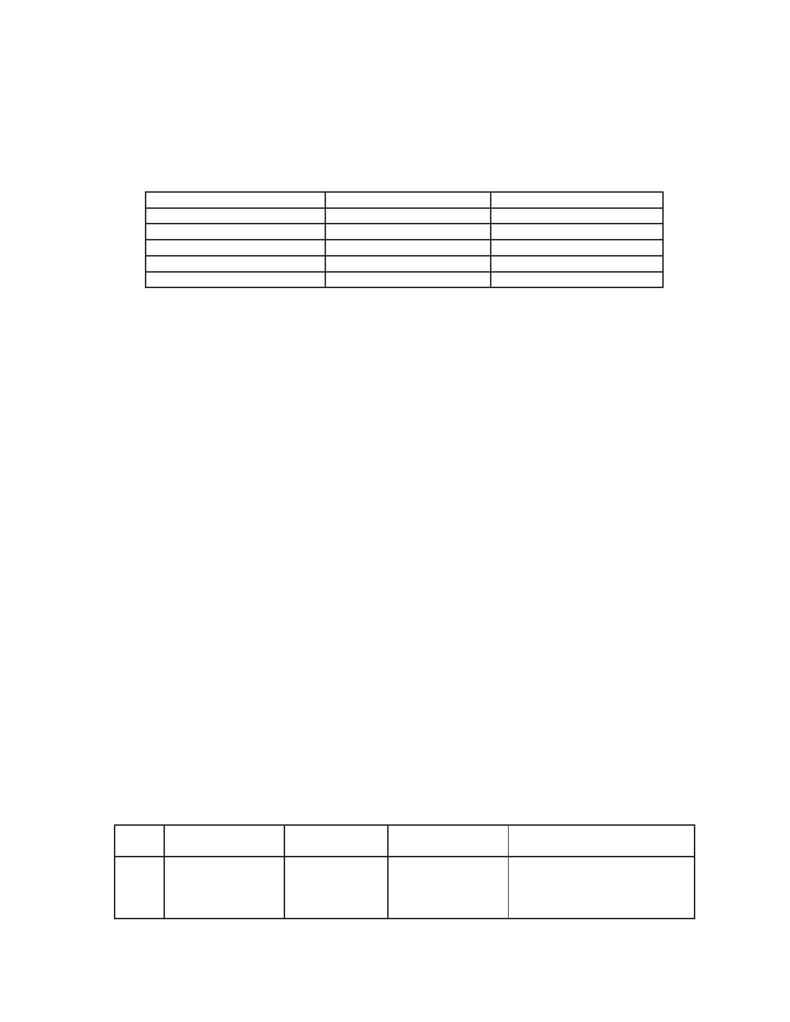
Table 1. Concentration calibration range of stream A and B
Components
Stream A-reactor
Stream B-still effluent
MCA (desired product)
40-80%
95-100%
DCA (undesired by-product)
1-4.5%
0-5%
Acetic acid
15-50%
0-4%
Acetyl chloride (catalyst)
0.7-5.5%
-
Chloroacetyl chloride
0.8-3.5%
-
Before installation of the Raman system, percent conversion of MCA is routinely estimated every two
hours, and catalyst content was measured only every eight hours. The limitations of the existing sampling
procedure are:
(i)
Potential of operator exposed to high toxic and corrosive liquid.
(ii)
Poor estimation of MCA conversion by checking the specific gravity of the mixture of MCA,
DCA and chloroacetyl chloride.
(iii)
Poor estimation of catalyst content by titration that summed the acetyl chloride and chloroacetyl
chloride.
(iv)
Time delay of one shift to detect the process changes that affect the catalyst level.
(v)
Difficulty in process optimization due to time-delay and inaccuracies of available analysis.
The installation of Multiplex Raman system has overcame the above mentioned limitations:
(i)
Sampling is remote from 200 m away using four fiber-optic lines for four sampling points.
(ii)
Improved accuracy of analysis after several calibrations to the spectra.
(iii)
No time delay, thus the process can be optimized and improved the yield of MCA.
In conclusion, the multiplexed optical fiber Raman system has provide the process with reliable real-time
data resulting in considerable cost savings and improved product purity, and has lowered the exposure of
operators to a toxic substance.
CURRENT AND FUTURE DEVEPLOPMENT
In the last ten years, instrumental advances have made Raman spectroscopy an attractive technique for
chemical analysis. Improved lasers, charge-coupled device (CCD) detectors, and wavelength separating
filters have increased the signal-to-noise and ease of use of this technique. In addition, the ability of the
technique to employ long-distance optical fibers and to view samples through glass windows has made it
particularly interesting to the process analyzer community. The application of Raman spectroscopy to the
monitoring of chemical processes has been reported by numerous workers in both laboratory and plant
process situation. Table 2 showed the list of successful Raman Spectroscopy system used as on-line
analyzer to improve the process control and optimization.
Table 2. Application of Raman Spectroscopy in on-line Process Monitoring
Year
Species-of-interest Reported by
Other methods
considered
Advantages of Raman
Spectroscopy
2004 Monochloroacetic
acid (liquid phase)
Weyer, et al.
MIR, autotitration,
GC, NIR, NMR
Uncontactable sampling
method, multiplexed sampling,
sufficient discriminatory
capability
