16
Enzyme Mediated Asymmetric Synthesis
"Kinetic Resolution and Chemoenzymatic Dynamic Kinetic Resolution of
Functionalized
-Hydroxy Amides"
Prepared by:Lee Siang Hua (HT033441Y)
Prepared for: Assoc. Prof. Marc Garland
Date of submission: 13 April 2005
Dept. of Chemical & Biomolecular Engineering,
National University of Singapore,
10, Kent Ridge Crescent, Singapore 119260
Email: g0302120@nus.edu.sg
BACKGROUND
The asymmetric synthesis of optically active compounds can be achieved in several ways, 1) resolution
of racemates 2) synthesis from the "chiral pool" 3) asymmetric induction using stoichiometric quantity of
chiral reagent 4) asymmetric chemical catalysis 5) enzyme-mediated processes. For asymmetric synthesis
the most important enzymes are oxidoreductases, hydrolases, lyases (catalyzing additions to double
bonds), and less generally, ligases (e.g. aldolases). Some of the enzymes and their functions are listed in
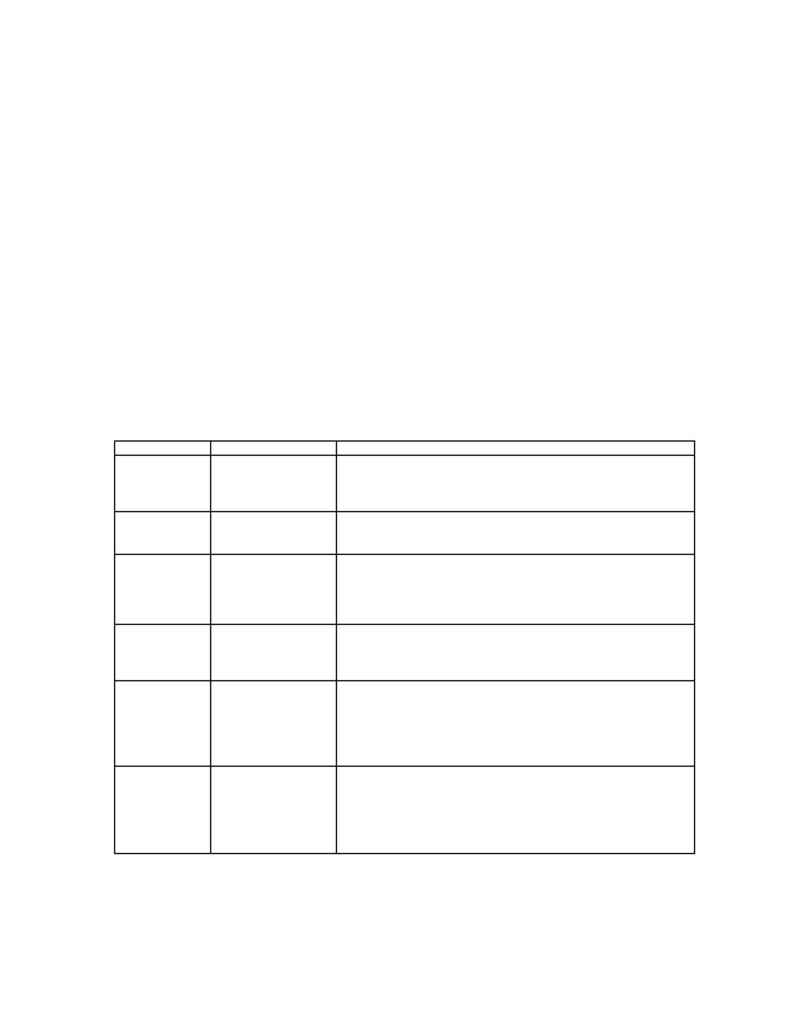
Table 1 (compiled from Ullman, Vol. A9, pp. 429-434).
Table 1. Enzymes for Asymmetric Synthesis
Enzymes
Functions
Examples of industrial application
Esterases
Amidases
Kinetic resolutions of
racemic mixtures
Pig liver esterase - asymmetric synthesis of chrysanthemic,
permethrinic, and caronic acids from the corresponding racemic
methyl esters
Chymotrypsin and acylase kinetic resolution of amino acids
Lipaces
Amidases
Hydrolysis
Hog pancreatic lipase enantioselective hydrolysis of glycidyl
butyrate (>95% ee.)
Amidases hydrolyze N-acylamino acids
Amidases
Formation of bonds in
polypeptides and
proteins
Trypsin-catalyzed reaction conversion of porcine insulin to human
insulin.
Thermolysin-catalyzed reaction synthesis of an aspartame precursor
Chymotrypsi, papain and trypsin total synthesis of dynorphin (an
oligopeptide)
Adolases Catalyze
the
cleavage
and formation of
carbon-carbon bonds
in carbohydrates
Fructose 1,6-diphosphate adolase synthesis of rare, non-natural and
isotopically labeled carbohydrates such as D-Fructose 6-Phosphate
and L-Sorbose.
Lyases
Hydrolases
Isomerase
Catalyzing additions
to double bonds
Isomerization
-amylase and glucamylase conversion of starch to glucose
Glucose isomerase isomerization of glucose to fructose
Aspartase production of aspartic acid
Fumarase production of malic acid from fumaric acid
Galactosidase synthesis of glycosides
Epoxy hydrolases open epoxides regiospecifically
Dehydrogenase
Reductase
reduction
Horse liver alcohol dehydrogenase stereoselective reduction of
ketone
Enoate reductase stereoselective reduction of
,-unsaturated
carbonyl compound to the saturated derivatives
Lactate dehydrogenase reduced
-oxo acids enantiospecifically
to
-hydroxy acids
The utilization of enzymes in organic synthesis can be advantageous for several reasons (Roberts et al:
1995). Firstly, enzymes catalyze reactions under mild conditions (37°C/1 atm/pH 7). The transformations
are often remarkably energy-efficient compared to chemical processes. Secondly, enzymes often promote
highly chemoselective, regioselective, and stereoselective reactions, and being chiral catalysts, they are
