14
The degradation rate is weakly dependant on temperature due to low decomposition free energy
(
E=8.37 kJ/mol). Increasing temperature decreases the solubility of O2 and thus decreases the
concentration of photogenerated holes, thus lower the degradation rate.
Effect of surface area
The photocatalytic degradation is affected by adsorption of the substrate on the catalyst, thus higher
surface area will promote degradation rate.
Catalyst reusability
The catalyst can be filtered and reuse for several times for most of the substrate. Catalyst saturated with
dye substrates is hardly regenerated. Salt will inhibit the catalyst activity and can be restored by washing
with water.
Relative rates of degradation
The degradation of organic compounds proceeds through progressive attachment of OH groups. Thus
compounds which facilitate the attachment of OH* radical will degrade faster. The benzene substituted
with functional groups contain an unshared pair of electrons will degrade from high to low in the
sequence of O
-
, NR
2
, NHR, OH, OR, NHCOR, OCOR, SR. Groups that lack of unshared electron, the
degradation rate will decrease in the sequence of N
R
3+
, NO
2
, CN, SO
3
H, CHO, COR, COOH, COOR,
CONH
2
, CCl
3
, NH
3
+
. Surfactants with aromatics rings are more easily degraded than those containing
only alkyl or alkoxylate groups. The decomposition rates of amphoteric surfactants are slower than
cationic and non-ionic surfactants. The decomposition of nitrogen moieties surfactants is lower and in the
following order: pyridine ring > secondary amine > tertiary amine > peptide > quaternary amine. Ring
contain nitrogen atoms will not degrade completely.
CURRENT AND FUTURE DEVEPLOPMENT
The area of photocatalysis has been growth explosively during the past ten years, particularly regarding
technology applications. The biggest challenge of photocatalytic degradation is the reactor design. Two
typical approaches are generally applied: 1. immobilized the TiO
2
to certain medium. 2. Mixed the TiO
2
particles together with wastewater and separate it from effluent after treatment. For the first approach,
several studies had been reported. Fujishima A. et. al. (1997) had developed the TiO
2
films on different
substrates such as tile and glass for indoor environmental clean-up. Hofstadler, K. et. al. (1994) reported
the reactor design of TiO
2
immobilized on fused silica glass fibers for wastewater treatment. The major
problem for first approach will be the robustness of the film and high cost of manufacturing. However,
for the second approach, the separation of TiO
2
particles has become an important issue. The classical
solid-liquid separation process, such as sedimentation of TiO
2
after pH adjustment or the coagulation
with flocculants like basic aluminium chloride, are not satisfactory because the sedimentation take too
long hours and after the sedimentation step the supernatant must be filtered.
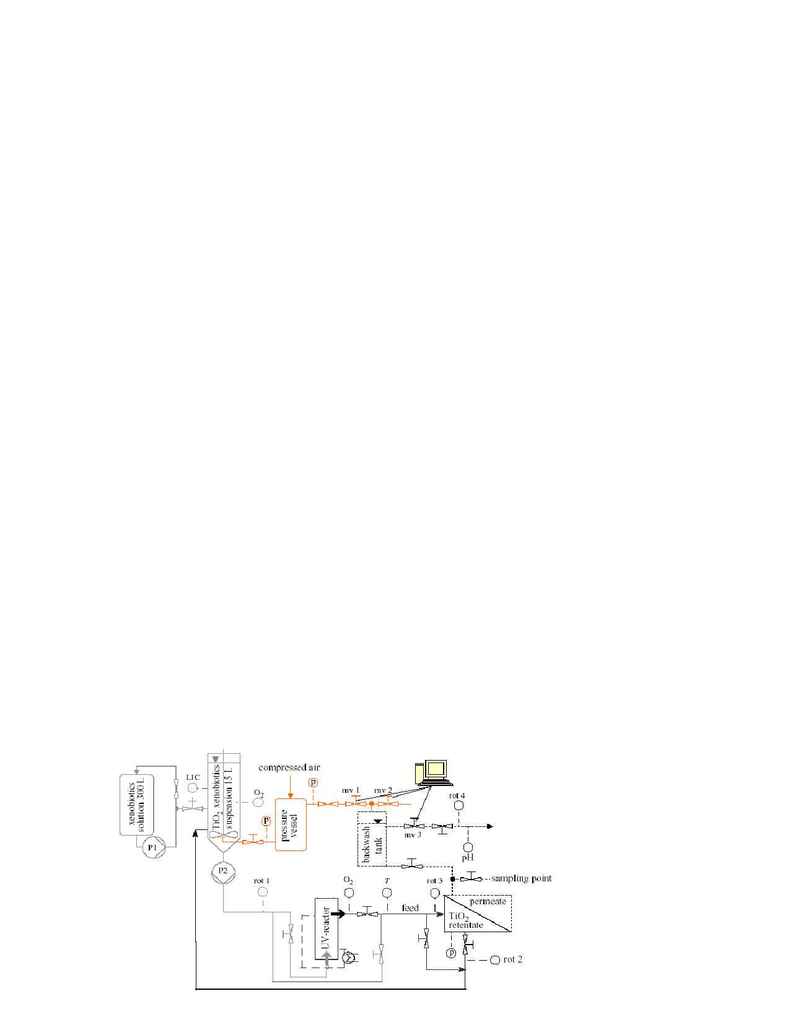
Doll and Frimmer (2005) reported a good approach of combining cross-flow microfiltration with
photocatalytic reactor to achieve long-term stability of the photocatalyst activity. The experiment had
been carried out in a pilot plant as shown in Figure 1.
P1 & P2: pumps 1 and 2
rot 1,2,3,4: rotameters 1,
2, 3 and 4:
p: manometer
mv 1,2,3: computer
controlled magnetic
valves 1, 2 and3
online data from the
sensors of pH,
temperature (T), and
dissolved oxygen (O2)
