13
scavenger or a surface defect state is available to trap the electron or hole, their recombination is
prevented and a subsequent redox reaction may occur. These will degrade the complex organic
compounds to simple organic or complete mineralization to CO2.
Compounds degradable
Almost all types of organic and inorganic substances can be degraded using photocatalysis. Table 1
shows the summary of compounds reported through out the years,
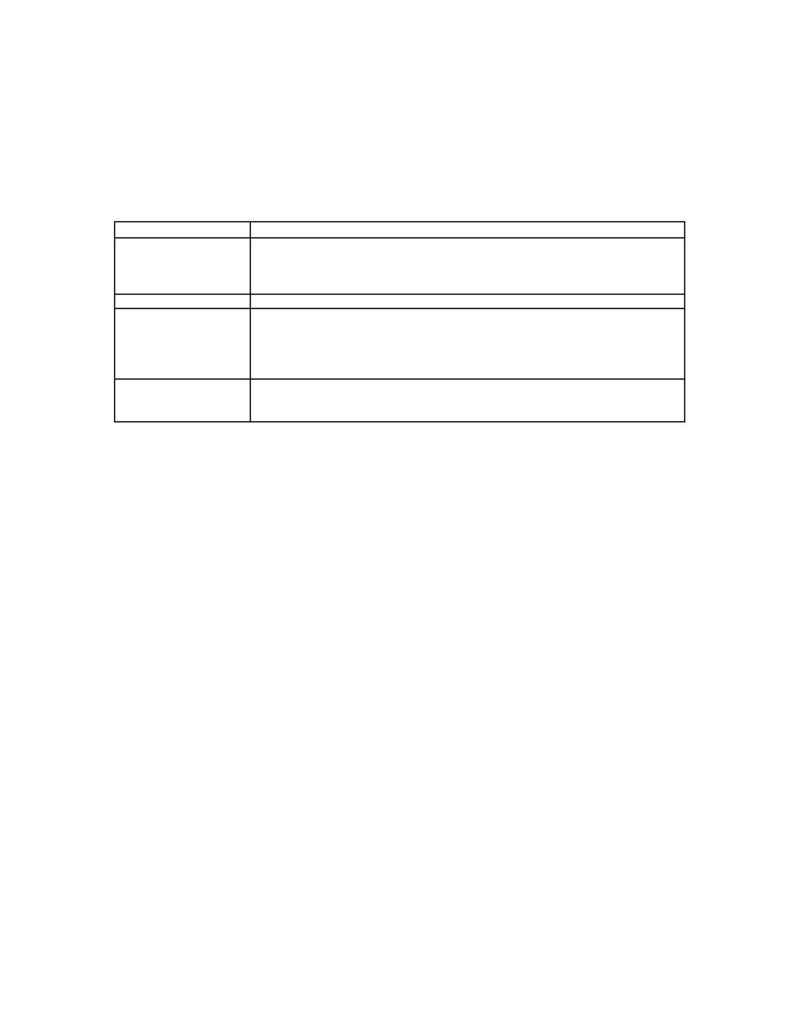
Table 1. Summary of compounds degraded by various researchers using photocatalyst
Groups Compounds
Apliphatic Gaseous
formaldehyde,
Formic
acid, CHCl3, CHBr3, CCl4, Chloroform,
Dichloromethane, Trichloroethylene, ethanol, 2-propanol, perchloroethylene,
dichloroethane, mono-, di- and trichloroacetic acid, MTBE, glycolic acid, citric acid,
monochlotophos
Inorganic AgNO3,
HgCl3,
CH3HgCl,
reduction of Cr(VI) to Cr(III)
Aromatics
Mallic acid, Benzene, Chlorobenzene, Nitrobenzene, Phenol, Toluene, Salicylic acid,
Benzoic acid, p-Hydroxybenzoic acid, 2-Chlorophenol, 4-Chlorophenol, 2,4-
Dichlorophenol, 3,5-Dichlorophenol, 2,4,6-Dichlorophenol, 2,3,5-Dichlorophenol,
Pentachlorophenol, 4-Nitrophenol, Phenoxyacetic acid, 2,4-Dichlorophenoxy acetic
acid, Octaphenylcyclo tetrasiloxane
Surfactant dyes
Textile dye reactive Black 5, Commercial azo dyes, Sodium dodecyl sulfate, Sodium
dodecyl benzene sulfonate, p-Nonylphenyl poly(oxyethylene) ether, Methylene blue,
Rhodamine B, Methyl orange, Fungicide metalaxyl
Effect of light intensity and wavelength
UV light provides the photons required for the electron transfer from valence band to conduction band of
the photocatalyst. The energy of a photon is related to its wavelength and the overall energy input is
dependent upon the light intensity. A light source of consistent intensity and particular wavelength is
desirable. The wavelength and intensity is dependent on the degraded compounds and catalyst dosage.
Effect of adsorption
The degradation of the substance depends on the adsorption of the substance on TiO
2
. The substances
which are adsorbed strongly degrade faster.
Effect of pH
Adsorption is at maximum near neutral pH. For weakly acidic substance, degradation increases at lower
pH. Some substances undergo hydrolysis at alkaline pH causes higher degradation. In alkaline pH, the
OH* radical is higher concentration and may cause higher degradation. For substances which dissociate
in certain pH range, the degradation rate will increase.
Effect on anions
Chloride anions decreased the degradation rate due to its adsorption to TiO
2
surface at low pH and strong
absorption of UV light. Nitrate anions had a negligible effect. Sulfate anions decreased the degradation
rate due to adsorption. Carbonate and bicarbonate anions react with OH* radicals thus reduce the
degradation rate.
Effect of cations
Fe
3+
cations increase the degradation rate at concentration lower than 0.5 mmol/dm
3
and retarded the
process at excessive concentration. The effect of Fe2+ cations is similar. Ag+ at concentrations of 0.1
mmol/dm
3
has similar effect. Cu2+ cations increased the degradation rate slightly at low concentration
(<0.01 mmol/dm
3
) and lower the rate at higher concentrations. The presence of cations in general has a
detrimental effect due to associated anions and the effect of salt on substrate adsorption.
Effect of temperature
