Kolmetz.com Thermo Paper Page 14
Conclusions
The results from these runs have shown that there will be variations between
thermodynamic packages within a simulator and the same thermodynamic package
between simulators. It is important to analyze the results for each system based on an
appropriate thermodynamic method associated with the simulation package.
Recommendations
An equation of state can be used over a wide range of temperatures and pressures,
including the subcritical and supercritical regions. Equations of state are typically used
for ideal or slightly non-ideal systems, thermodynamic properties for both the vapor and
liquid phases can be computed with a minimum amount of component data. Equations of
state are suitable for modeling hydrocarbon systems with light gases. Equations of state
are not capable of properly representing highly non-ideal systems, such as alcohol and
water systems
For the best representation of non-ideal systems an activity coefficient model may be the
best. One draw back of an activity coefficient model is that you may need to obtain
binary interaction parameters from regression of experimental vapor-liquid equilibrium
(VLE) data.
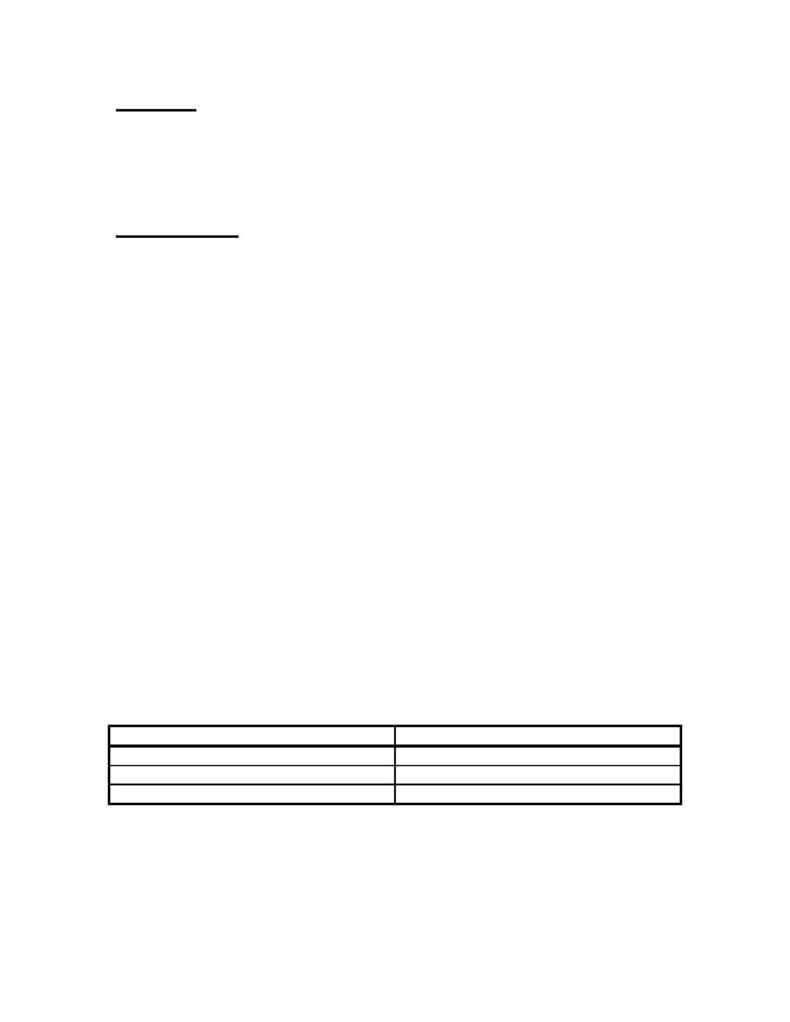
Table 5 provides some choices for deciding which thermodynamic model to try when
modeling distillation columns. There are a few things to keep in mind when looking at
this chart. Non-polar fluids may be modeled with an equation of state. Polar fluids are
best modeled with a fitted activity coefficient model. Some systems have specific
thermodynamic models designed especially for that kind of system. For example, there
are some thermodynamic packages that specifically model electrolytic solutions. There
are other thermodynamic packages that model alcohol systems and amine systems.
These facts should be taken into account when trying to choose a thermodynamic model.
(3)
Table 5
Thermodynamic Model Selection Table (5)
System Thermodynamic
Model
All Gases, Non-Polar Solutions
Peng Robinson, SRK
Moderately Non-Ideal, Polar Solutions
NRTL, Wilson, Van Laar
Highly Non-Ideal, Polar Systems
NRTL, UNIQUAC
***Note*** When using the following equations, NRTL, Wilson, and Van Laar, you
should make sure that all of the Binary Interaction Parameters are present before using
the equation to obtain the best results. (5)
14
