Amersham Bioprocess 11000846 Page 8
8
Downstream
thirty seven
A4
A5
OD
280
CYCLE
1
2
3
4
5
6
Vc
0
OD
280nm
1.2
1.0
0.8
0.6
0.4
0.2
A10
5
10
Vc
OD
280nm
1.2
1.0
0.8
0.6
0.4
0.2
A8
10
20
30
40
50
Vc
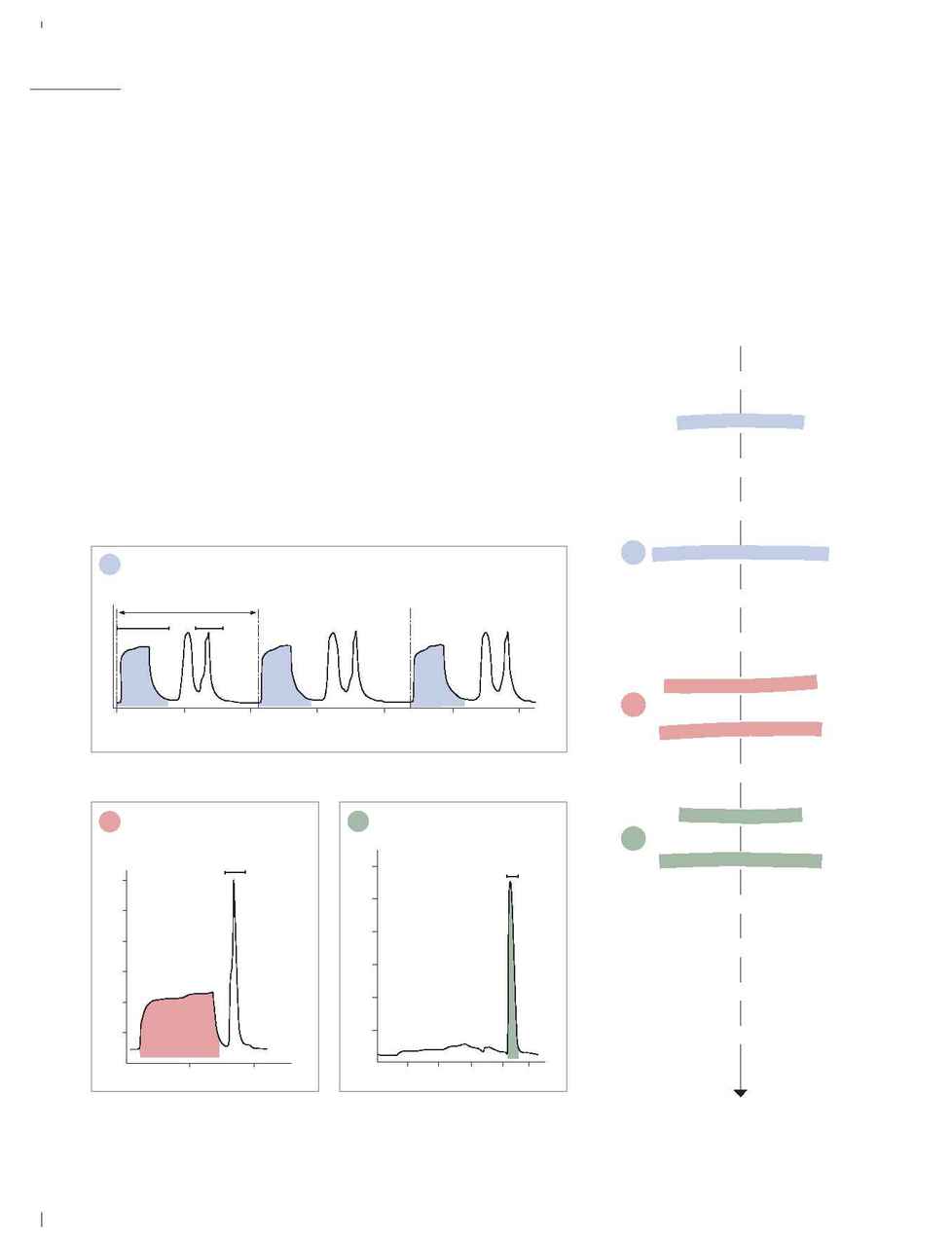
Anion exchange chromatography on DEAE Sepharose Fast Flow.
Anion exchange chromatography on
Q Sepharose Fast Flow.
Cation exchange chromatography on
CM Sepharose Fast Flow.
Patent for chromatographic IgG
production
P
atent number EP 1 268 551 B1 has been granted for a chromatographic
method producing IgG. The method has been developed by Amersham
Biosciences and begins initially as part of the chromatographic process for
albumin. After passing through the DEAE SepharoseTM column, the IgG fraction
then begins its separate process, with purification on Q Sepharose Fast Flow
followed by two steps on CM Sepharose Fast Flow. This process results in IgG
preparations that are more than 99% pure and conatin less than 1% of
aggregates. The level of prekallikrein activity is less than 10 IU/ml, well below the
European pharmacopoeia limit of 35 IU/ml.
RECOVERED PLASMA
Pretreatment
Sephadex G25 C
Euglobulin precipitation
Centrifugation
DEAE Sepharose Fast Flow
Ultrafiltration
pH- and I-adjustment
Q Sepharose Fast Flow
CM Sepharose Fast Flow
Ultrafiltration
Solvent/detergent
CM Sepharose Fast Flow
Ultrafiltration
Formulation
Sterile filtration
Filling
IgG
A
B
C
A
B
C
