17
Downstream
thirty seven
Were there challenges converting to STREAMLINE
and how did you overcome them?
"The main problem to overcome was clogging of the
adsorbent material and adaptor nets due to DNA and cell
debris. The solution has been to remove cells in the harvest
by using an in-line cell trap and pre-filtration by a 10 µm
dead-end filter.
Another challenge was to adapt and program the ÄKTApilot
system for STREAMLINE as there was no kit and strategy
developed for EBA. And then there was the possibility to
include in-line desalting.
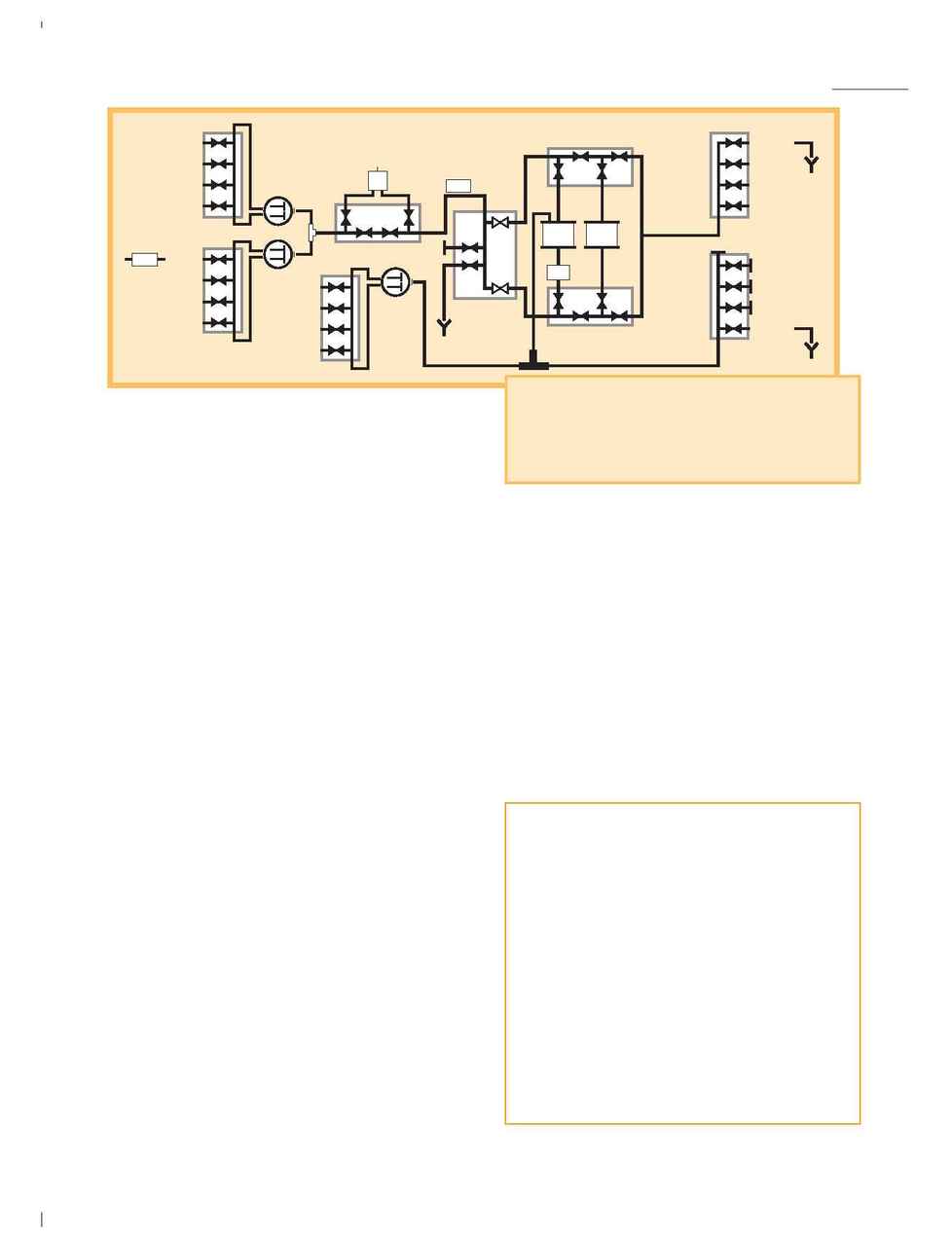
We adapted the flow scheme according to the diagram in
Figure 1. The "sample pump" was used to pump the
hydraulic liquid to move the adaptor down. Elution is done
in packed mode, so reverse flow was required. Sample was
introduced via one of the system pumps. In addition, we
needed to separate the outlet valves to create a sample pump
outlet. Programming was challenging but successful. Much
support was given by Amersham Biosciences."
How did you connect the second step to
automate the process?
"We installed a second UV monitor (see Figure 1, UV-4) so
that it was possible to detect the protein peak from the
STREAMLINE column and apply it directly onto the
desalting column. We accomplished this using a watch
function for the UV-signal in UNICORN. Basically no special
problems were encountered and today the whole run (affinity
coupled to desalting) is fully automated."
Do you use any special cleaning protocols?
"Our cleaning procedure is based on the one recommended
by Amersham Biosciences, but we omit the ethanol and the
acetic acid due to the formation of precipitates, especially in
combination with sarcosyl."
General info about bioMerieux:
bioMerieux bv (the Netherlands) is an R&D and production site,
specialized in research, manufacturing and marketing of
diagnostic systems and kits.
bioMerieux bv, as part of the bioMerieux sa group, has a quality
system that is in compliance with ISO 9001, ISO 13485, FDA
quality systems and regulations and the IVD directive 98/79/EC.
Within the Biomaterial Development & Production group of
bioMerieux bv, a broad range of biomaterials (like monoclonal
antibodies, polyclonal antibodies and antigens) are developed
and produced for bioMerieux's own production and R&D.
Additionally, biomaterials are supplied to other companies over
the world. Contract manufacturing is a growing market within
bioMerieux bv.
For more information about bioMerieux bv and biomaterial
contract manufacturing, please visit: www.biomerieux.com.
What are the overall benefits of this approach?
"This approach has enabled us to handle large volumes cost
effectively. After the pre-filtration step, the whole
purification is fully automated. The current process was first
developed on a STREAMLINE 25 column coupled to an
ÄKTAexplorerTM system. The transfer of the methods to
ÄKTApilot and STREAMLINE 100 column was
straightforward. Total development time, including cell
culture and CIP, was about 5 months."
This process has now been in use for several months and
bioMérieux Boxtel are clearly pleased with benefits gained by
the smooth transfer to this solution.
V1
V2
V3
V4
V5
V6
V7
V9
V8
Inlet A1
Pump A
1
2
4
3
10
6 7 8 9
5
Pump B
Sample
Pump
Column
1
UV 4
Column
2
Inlet A2
Inlet A3
Inlet A4
Waste F1
Outlet F2
Outlet F3
Outlet F4
Waste F8
Waste 2
Inlet B1
AS 2
AS 1
Inlet B2
Inlet B3
Inlet B4
Sample 1
Sample 2
Sample 3
Sample 4
1
Flow AB
0.0 ml/min
7
pH
7.33
2
Press 1
0.00 MPa
8
Cond
0.331 mS/cm
3
Press 2
0.03 MPa
9
UV 1
621.005 mAU
4
Sample Flow
0.0 ml/min
UV 2
646.289 mAU
5
Press 3
0.00 MPa
UV 3
2101.42 mAU
6
Press 4
0.03 MPa
10
UV 4
A
280
mAU
Fig. 1. Line diagram showing the configuration details in UNICORN to run
STREAMLINE expanded bed adsorption.
