Unit-Specific Pharmacists
Vol. 28 No. 1 · January 2003 ·
P&T
®
53
was evident, and they, too, shared in the hos-
pital-wide mission to prevent medication er-
rors. The medical staff and members of the
P&T committee and the hospital's medical
board listened to our plans and accepted our
goals as presented.
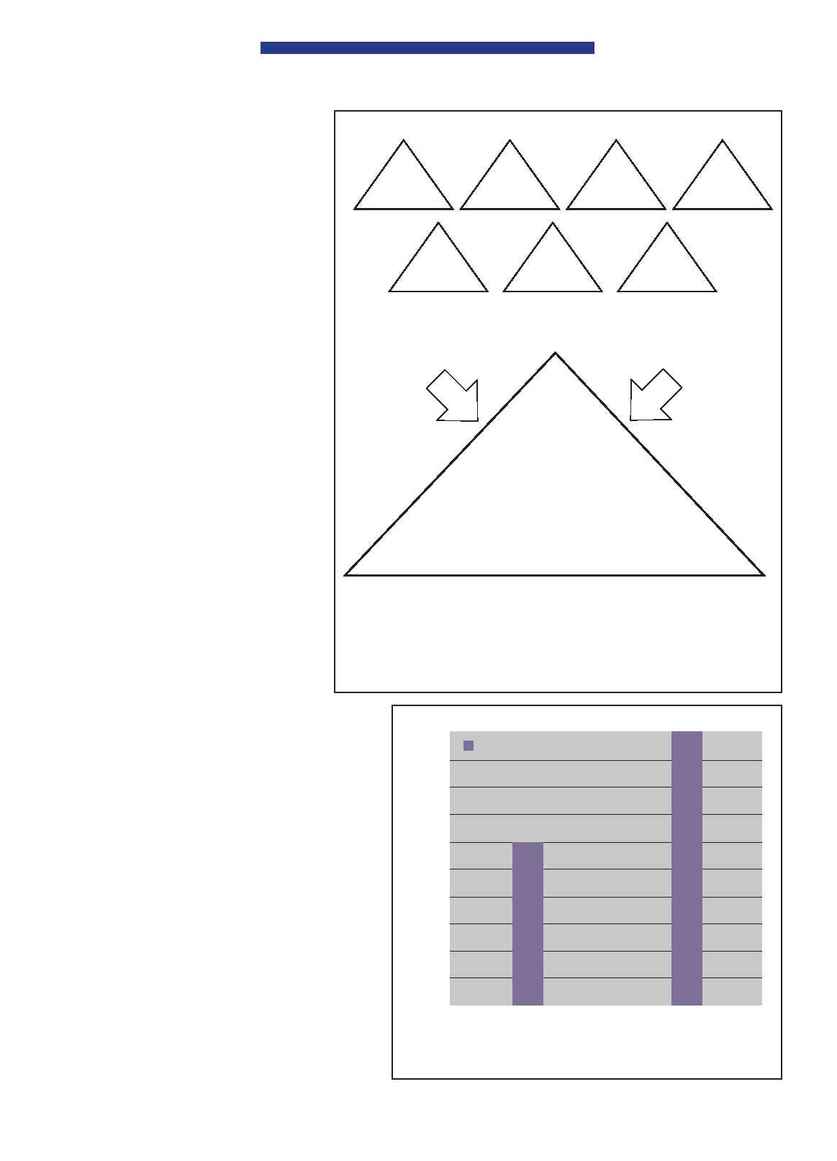
The reporting of accumulated adverse drug
reactions (Figure 3) is ongoing. All reports are
funneled to the clinical pharmacist for research
and follow-up. We have re-educated our pro-
fessional staff on the importance of docu-
menting adverse drug reactions. We promoted
error-reporting as an educational tool, which
was an integral part of the continuum of patient
care and safety. The pharmacist has thus be-
come the first line of defense in the process of
defining and documenting adverse drug reac-
tions and other intervention data.
The data accumulation for the intravenous-
to-oral conversions occurs several times per
week as the unit-specific pharmacist makes
rounds with pharmacy computer-generated
drug profiles. This allows the pharmacist to
categorize the five medications approved by
the P&T committee for automatic intravenous-
to-oral conversion (Table 1) and to identify the
patients who are receiving them. The phar-
macist can then refer to the patient's chart to
see whether the patient profiles satisfy the pre-
approved criteria, and the change can then be
noted on the chart.
Decreasing the dosage of some medications
that affect the renal system, such as nizatidine
and the quinolones, is accomplished in a sim-
ilar way. The pharmacy computer generates
profiles of drug usage to quickly highlight the
changes needed. The pharmacist then makes the nec-
essary entry into the patient's chart if the patient quali-
fies for a change in drug dosing.
REPORTING OF OUTCOMES
In a hospital environment, performance measurement
and its proper organization and communication are cru-
cial to any new performance improvement initiative. Our
pharmacist intervention indicators were changed to re-
flect those areas that our clinical pharmacists consid-
ered to be problematic; interestingly enough, these areas
had not been noticed before but they arose from obser-
vations and early experiences on the patient-care units.
Pharmacy intervention indicators are opportunities for
pharmacists to intervene, in a timely fashion, in pre-
scribed ordering and patient care. These indicators in-
clude allergies, order legibility, drug interactions, dose
adjustments, drug information, dosages, frequency and
usage of medication, missing medications, nonformu-
lary drug requests, and switches from intravenous to
oral medications.
We first identified useful methodologies for conduct-
ing outcomes assessments by reviewing the literature
Allergies
Dose
Frequency
Usage
Drug
Information
Interactions
Incompatibility
Order
Clarification
Nonformulary
Drug requests
Allergies
Order legibility
Drug interactions
Renal adjustment
Drug information
Dosagefrequencyusage
Missing medications
Nonformulary drug requests
IV to PO medication changes
IV
Solutions
Unit-Specific Pharmacist Program
Figure 2
Evolution of intervention indicators. As the unit-specific pharmacist
program evolved, the opportunity to intervene became more apparent because
the pharmacist could focus entirely on pharmaceutical care as a part of the
multidisciplinary team.
100
90
80
70
60
50
40
30
20
10
0
March 2000 to
February 2001
Adverse drug reactions
60
March 2001 to
February 2002
Number of reports
100
Figure 3 Reports of adverse drug reactions.
